has never been easier
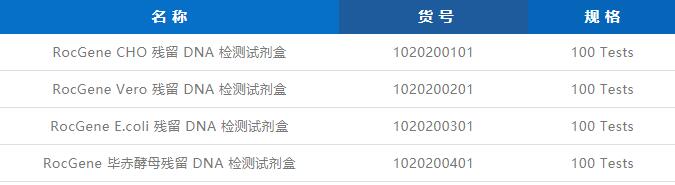
Biopharmaceutical products, whether they are antibodies, vaccines or cell gene therapy drugs, usually require host cells as carriers in their production process, such as CHO, E.coli and Vero cells. Since the DNA released from host cell lysis has potential risks of tumorigenicity, mutagenesis, and immunogenicity, it is necessary to adopt effective removal methods in the downstream purification process, and control the residual amount within the scope of regulations.

As the 2020 edition of the Chinese Pharmacopoeia officially included the quantitative PCR method for the detection of residual DNA, it was quickly adopted due to its advantages of high sensitivity, good reproducibility and ease of operation compared with DNA probe hybridization and fluorescent staining methods. used by everyone.
But people's pursuit of good products is endless. With the popularization of use, people gradually have higher requirements for residual DNA detection. Could the extraction and detection steps be simpler? Can the detection time be faster? Is there a more efficient way to avoid the PCR contamination problem?

Host Residual DNA Detection Solutions
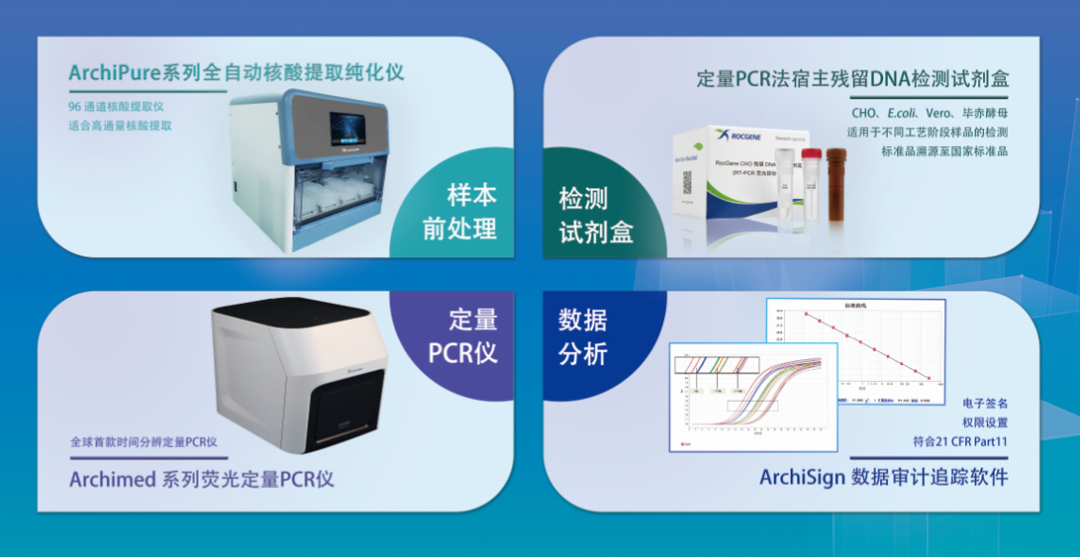
Based on the above requirements, Rocgene has developed a complete set of residual DNA detection solutions, currently covering four host cells, CHO, E. Series of quantitative PCR instruments and many models such as 7500, StepOne Plus and CFX96.
Solution Features
many
For the sample pretreatment step, we use the principle reagent of magnetic bead method, and provide a magnetic frame and an automatic nucleic acid extraction instrument to meet different flux requirements. When the sample volume is relatively small, a magnetic stand can be selected for manual extraction, but the extraction volume of residual DNA samples is generally larger, especially in the process optimization stage, so we usually need extraction equipment with larger throughput. The ArchiPure 96H automatic nucleic acid extraction instrument can complete up to 96 wells of extraction in a single time, and can eliminate the tedious manual operation steps to meet the experimental needs.

ArchiPure 96H全自动核酸提取纯化仪
Quick
The residual DNA amplification detection only takes 60 minutes, which is 1/3 faster than similar products in the market, allowing you to obtain the detection results one step faster.
It is good
The kit standard products are traceable to the national standard products of the China Inspection Institute, and the determination value is accurate and stable.
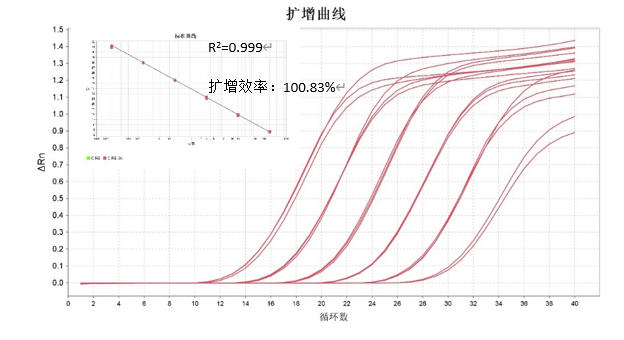
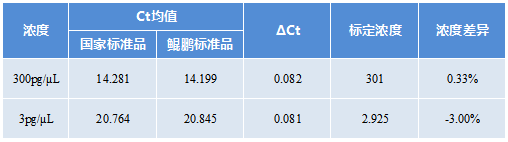
CHO Residual DNA Detection Kit Standard
Traceable to national standard products
The reagent contains UNG enzyme + dUTP system, which can effectively prevent PCR aerosol pollution. The UNG enzyme and dUTP in the reaction reagent can degrade the aerosol pollution from the previous amplification without affecting the amplification and detection of the sample DNA in this experiment. It is simple and convenient to use, just add the reaction program of 37℃ for 2min to the normal PCR reaction program.
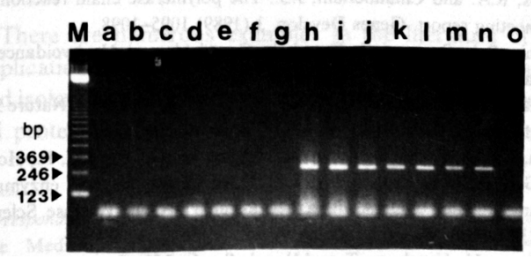
The dUTP-containing PCR amplification product (10 ng-10 fg) was added to a new PCR reaction system to simulate amplification product contamination.
a~g: with UNG enzyme; h~n: without UNG enzyme; o: no amplification product added
Province
The primer probe and enzyme mixture are pre-mixed into one tube, which can be directly added without reagent loss, which is more convenient to use
Product information